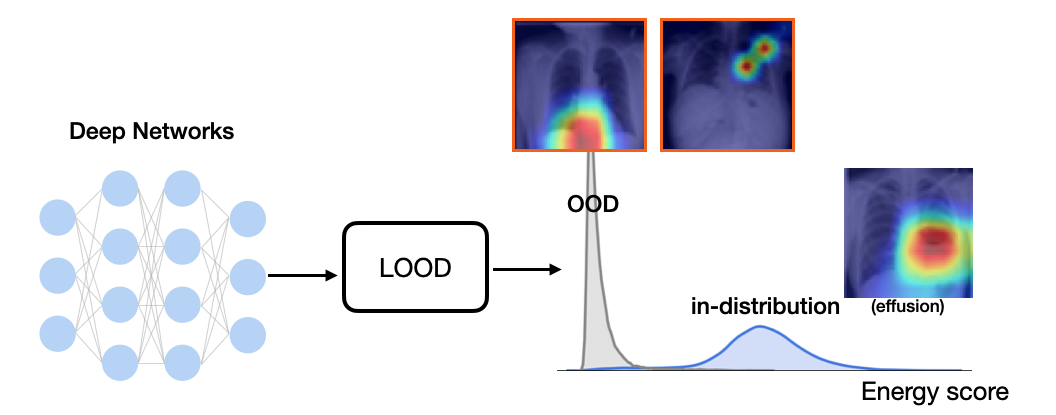
Pomemben napredek v kvantitativni znanosti o podatkih, zlasti pri uporabi naprednih tehnik računalniškega vida, je privedel do pomembnega in hitrega premika področja medicinskega slikanja k kvantitativni analizi in personalizirani medicini. V varnostno kritičnih scenarijih, kjer se sprejemajo klinične odločitve, je nujno, da modeli umetne inteligence (AI) vključujejo trdna jamstva za varnost in zanesljivost. En kritičen vidik ohranjanja varnosti je odkrivanje podatkov izven porazdelitve (OOD). Vhodni OOD podatki, ki se razlikujejo od učnih podatkov, lahko povzročijo kritične nevarnosti in napačne klinične odločitve s škodljivimi posledicami za bolnike. Obstoječe rešitve za odkrivanje OOD niso izvedljive za izzive v zapletenem zdravstvenem slikanju, zato ostaja izziv lokalizacija nepravilnosti in analiza pristranskosti modela.